Boosting Structural Stability in Zn-Ion Batteries thanks to Zn-Substituted Cathodes
|Aqueous zinc-ion batteries have emerged as promising sustainable energy sources thanks to their safety, low cost, and environmental friendliness. Among the various cathode materials investigated, Prussian Blue Analogues (PBAs) — particularly manganese hexacyanoferrate (MnHCF) — stand out due to their good theoretical capacity and sustainable redox chemistry. Yet, their use is limited by structural instability and compositional degradation during electrochemical cycling.
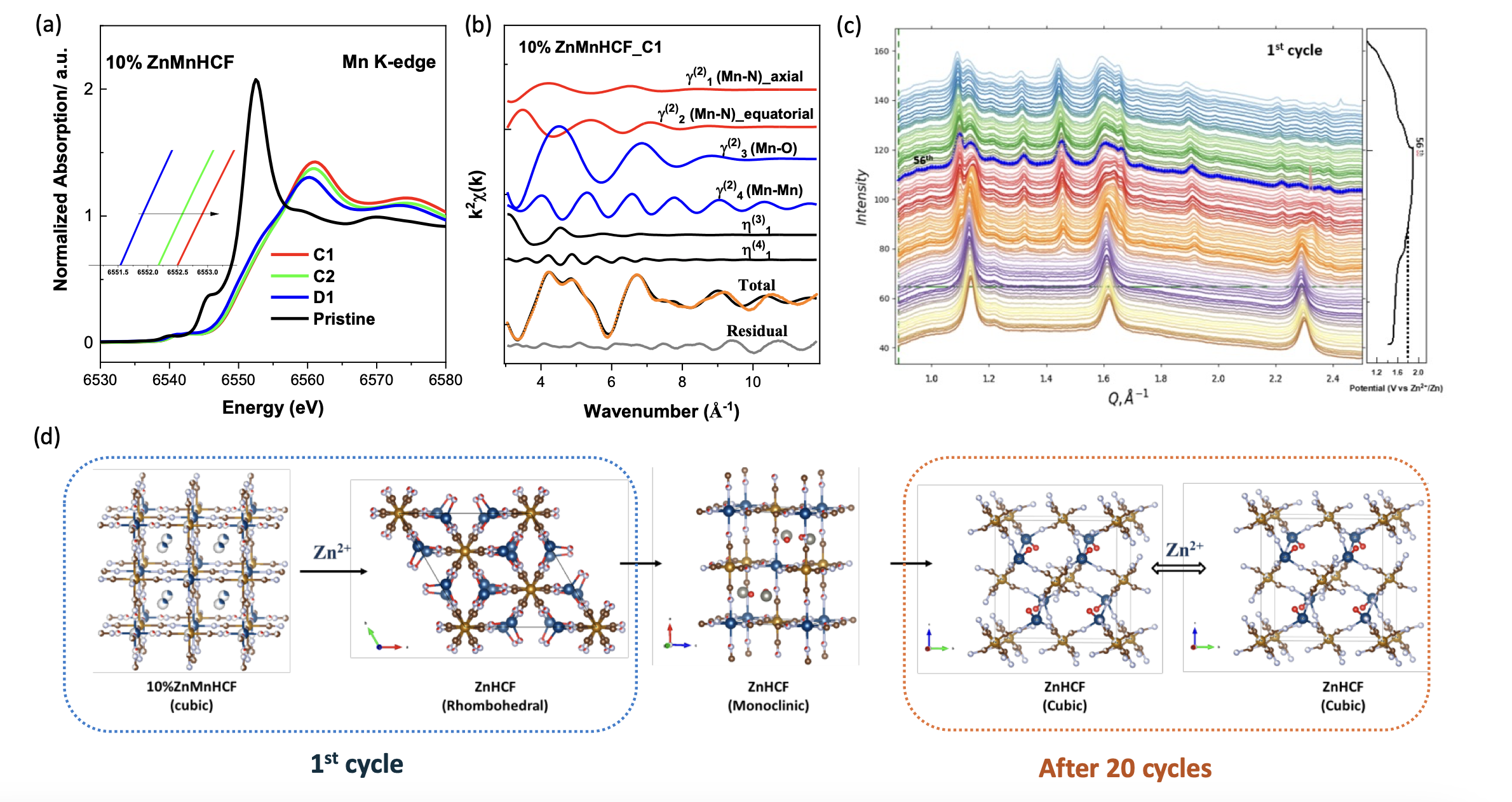
Dr Min Li, Dr Mariam Maisuradze (former CERIC PhD student), Prof. Marco Giorgetti and colleagues of the University of Bologna explored a novel approach to mitigate these limitations by synthesizing Zn-substituted MnHCF materials, introducing 3%, 10%, and 35% Zn into the MnHCF framework. Then, scientists characterised their electrochemical behavior and structural evolution using advanced analytical techniques, including X-ray Diffraction and X-ray Absorption Spectroscopy, available at CERIC Italian Partner Facility.
The Zn-substituted electrodes, in particular the 10% Zn-substituted MnHCF, showed a marked improvement in phase stability even after 100 cycles, due to an early transformation from cubic to rhombohedral and then monoclinic structures. Moreover, X-ray absorption spectroscopy revealed the formation of a Mn–O₆ framework post first-cycle charging, which remained stable through cycling — a direct indication of enhanced structural robustness.
These findings suggest that partial Zn substitution can fine-tune the delicate balance between structural stability and energy capacity in MnHCF-based AZIB cathodes, offering a pathway to more durable, scalable, and commercially viable aqueous Zn-ion batteries, even applying hybrid strategies that combine substitution with nanostructure design or protective coating.
ORIGINAL ARTICLE:



