A novel combined characterization study of anode materials for Na-ion batteries
|Sodium-ion batteries are a promising alternative to widely used lithium-ion ones, because they show high energy density, improved safety and lower environmental impact. For this kind of devices, the choice of anode material is crucial, since it has an impact on the battery’s performance and stability, as well as on the formation of the so-called solid electrolyte interface.
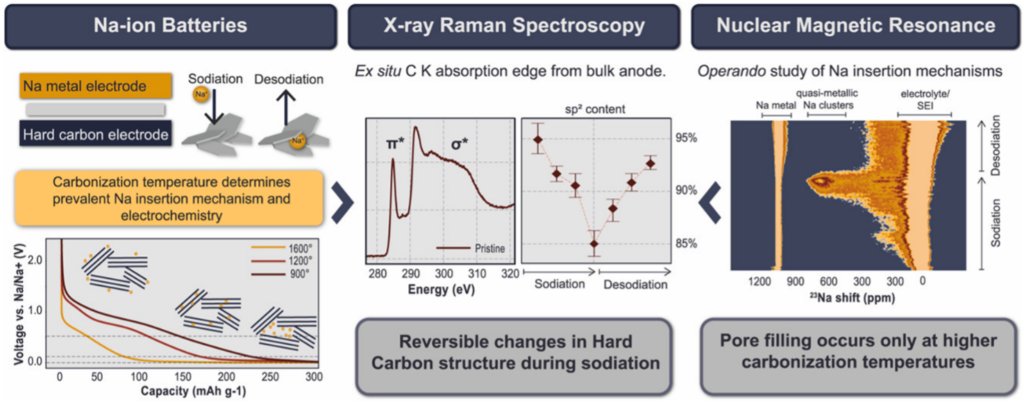
In the latest years hard carbons (i.e. non-graphitizable carbons), emerged as leading candidates for anode material in sodium-ion battery. The CERIC PhD candidate Matej Gabrijelčič, supervisor Dr Alen Vižintin and colleagues of the National Institute of Chemistry in Ljubljana, in collaboration with the Institute Jožef Stefan and University of Nova Gorica studied, using X-ray Raman spectroscopy (XRS), the structural changes of hard carbon negative electrodes (anode) in sodium-ion batteries induced by different carbonization temperatures and insertion of Na+ ions during sodiation. With XRS, they found that higher carbonization temperatures increase graphite-like order, while sodiation disrupts this order due to inserted Na+ ions, and that systematic variations in the solid electrolyte interface (SEI) composition occur during cycling. Moreover, Na insertion mechanisms and SEI composition in the structure of hard carbon negative electrodes have been confirmed by operando and ex-situ solid-state nuclear magnetic resonance (ssNMR) studies, performed with the NIKA and MAGIC Spectrometers available at the Slovenian CERIC Partner Facility. With ssNMR, they found that pore filling occurs mainly at higher carbonization temperatures.
Hopefully these findings will help to design more efficient, stable and long-lasting sodium-ion batteries.
ORIGINAL ARTICLE:



