Self-Transforming Nickel Pre-catalyst Powers Hydrogen From Urea
|Urea electrolysis is an emerging energy conversion technology that uses the oxidation of urea, commonly found in wastewater—as a low-energy pathway for hydrogen generation. Because urea breaks down at a much lower voltage than water, this process can significantly reduce the energy required for hydrogen production while simultaneously treating urea-rich waste streams.
In this context, a research team led by Dr. Neena S. John and Ph.D student, Mr. Nikhil N Rao at the Centre for Nano and Soft Matter Sciences (CeNS),Bengaluru, an autonomous institute under Department of Science and Technology (DST, Govt. of India) has developed a nickel hydrazine chloride “pre-catalyst” that transforms itself into a highly active material during electrolysis. This self-generated active phase enables efficient hydrogen production from urea-containing solutions.
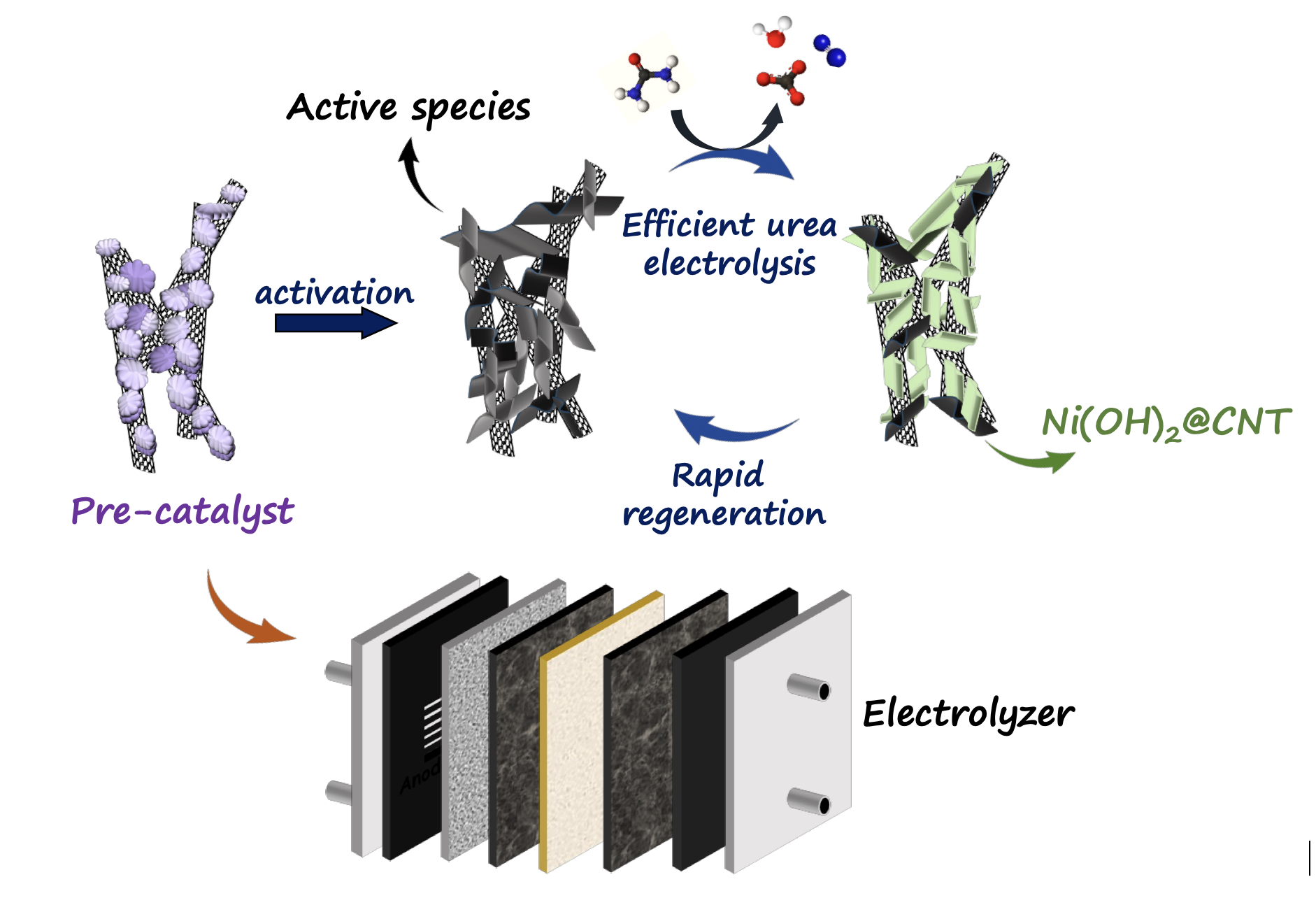
Under operating conditions, the catalyst reconstructs into a highly efficient active phase that shows excellent electrocatalytic activity and long-term stability. Using CERIC-supported access and in collaboration with Dr. Peter Kúš at the Czech partner facility, Hydrogen Technology Centre (HTC), Charles University, Prague, the team successfully implemented these advanced pre-catalysts in state-of-the-art anion-exchange membrane electrolyser systems. These device-level studies demonstrated the catalyst’s strong performance in a realistic operational environment.
The combination of in situ characterisation methods and advanced electrolyser testing highlights the significant potential of these nickel-based pre-catalysts to enhance hydrogen generation through urea-assisted water electrolysis.



