Describing low-temperature CO oxidation on platinum-ceria-based catalysts
|CO oxidation is an important reaction in preventing the emission of toxic fumes into the environment. Low-temperature CO oxidation is particularly desirable for the conversion of CO emitted by gasoline-powered vehicles during a cold engine start. Currently, the most efficient catalysts for low-temperature CO oxidation are platinum (Pt) and ceria (CeO2) containing compounds. However, the nature of the active sites and the mechanism of low-temperature CO oxidation on the Pt–CeO2 catalyst are still under debate.
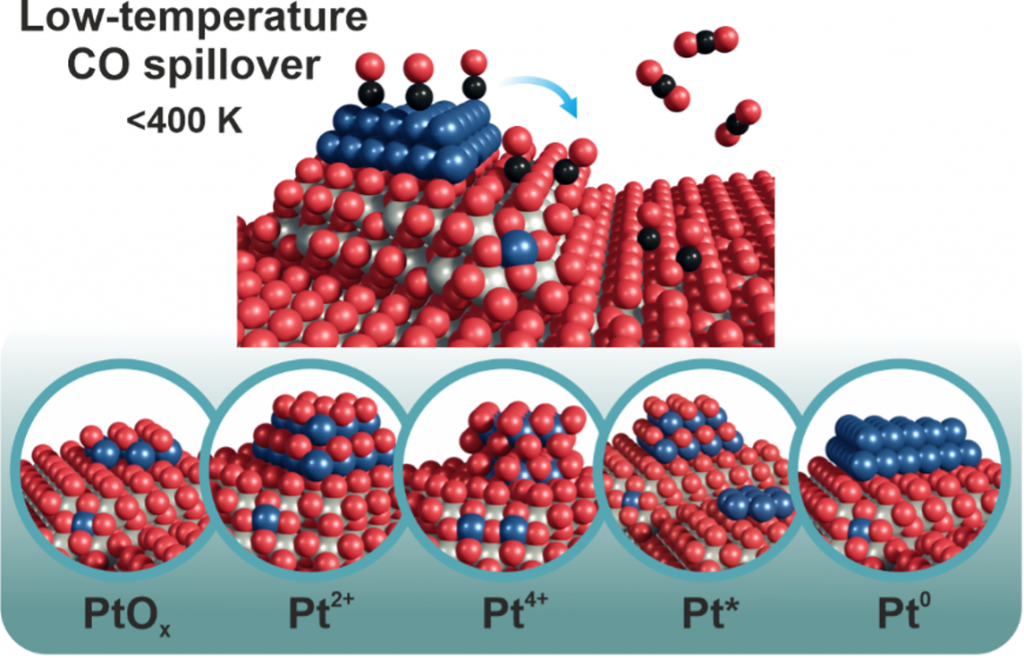
Alexander Simanenko, Dr. Yaroslava Lykhach, and Prof. Dr. Jörg Libuda from the Friedrich-Alexander-Universität Erlangen-Nürnberg, in close cooperation with colleagues from the Materials Science Beamline at Elettra and Czech CERIC partner facility at the Charles University in Prague, analyzed the redox capacity of Pt–CeO2 catalysts for low-temperature CO oxidation using synchrotron radiation photoelectron spectroscopy, resonant photoemission spectroscopy, and near-ambient pressure X-ray photoelectron spectroscopy. Various Pt–CeO2 systems were prepared containing specific Pt species that differ in oxidation state, chemical environment, and nuclearity. The oxidation states of the Pt species and Ce cations were monitored upon CO exposure as a function of temperature. The researchers found that the redox capacity for low-temperature CO oxidation was only observed for the Pt–CeO2 catalyst containing metallic Pt0 nanoparticles. In this system, the corresponding redox pathway is associated with CO spillover and the formation of bidentate carbonate species.
This study demonstrates that the nature of the Pt species in the Pt–CeO2 catalysts controls the mechanisms of low-temperature CO oxidation. These findings provide the basis for knowledge-based design of catalysts with tunable catalytic activity.
ORIGINAL ARTICLE:



