Stable and powerful fuel cells with rare earth metals alloy catalysts
|In the path towards a climate-neutral European Union, to be achieved by 2050, the development and the large-scale deployment of energy technologies is fundamental. Next to batteries, fuel cells are an important asset to achieve this result. Thanks to their properties, Proton Exchange Membrane Fuel Cells (PEMFC) have a great potential to become part of our daily life. Among their advantages, high power density and low operational temperatures make them a suitable energy source for transportation, from cars to public buses. However, the aspect preventing this technology’s diffusion is the catalyst material, mostly a rare metal such as platinum. Substituting or reducing the amount of platinum would make this technology more affordable and thus closer to a large-scale application.
In this regard, an example is given by the use of platinum alloys with rare earth metals (REM), such as yttrium (Y), gadolinium (Gd), and terbium (Tb). A collaboration among scientists from the KTH Royal Institute of Technology (Sweden), Chalmers University of Technology (Sweden) and the Charles University of Prague (Czech Republic) resulted in a publication by Dr Björn Eriksson (KTH) and colleagues that reports interesting performances by PtREM alloy catalysts under operational conditions.
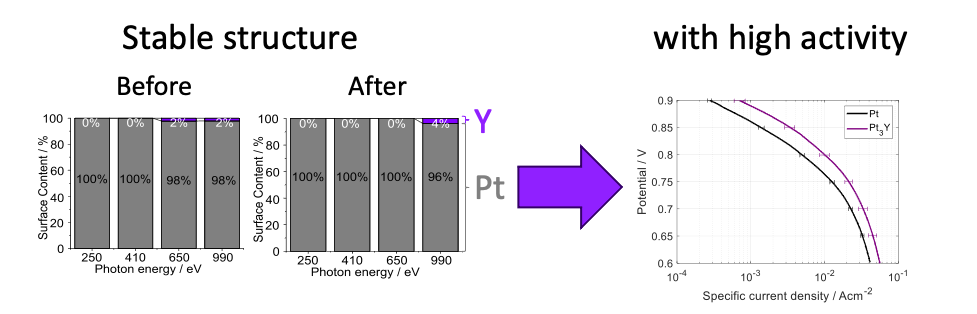
Synchrotron Radiation Photoelectron Spectroscopy (SRXPS) measurements, made at the Materials Science Beamline (MSB), available at the CERIC Austrian Partner Facility (Charles University Prague) at Elettra Sincrotrone Trieste, allowed the study of the surface composition of different PtREM alloys tested in this research work. These experiments showed that Pt3Y alloy had no significant change in the surface composition during operations, suggesting that this alloy is the most stable. Experiments also highlighted that Pt3Y and Pt5Gd have a specific activity that is 2.5 times higher than pure platinum. The combination of increased activities and low changes in surface composition, achieved in an operating fuel cell, shows that PtREM catalysts are a promising cathode material for PEMFC.
ORIGINAL ARTICLE:
-
02.04.2024 | Catalysis, Chemistry
Fabrication of fluorescent MOF micropatterns



