Unraveling the the secrets of squids’ camouflage abilities
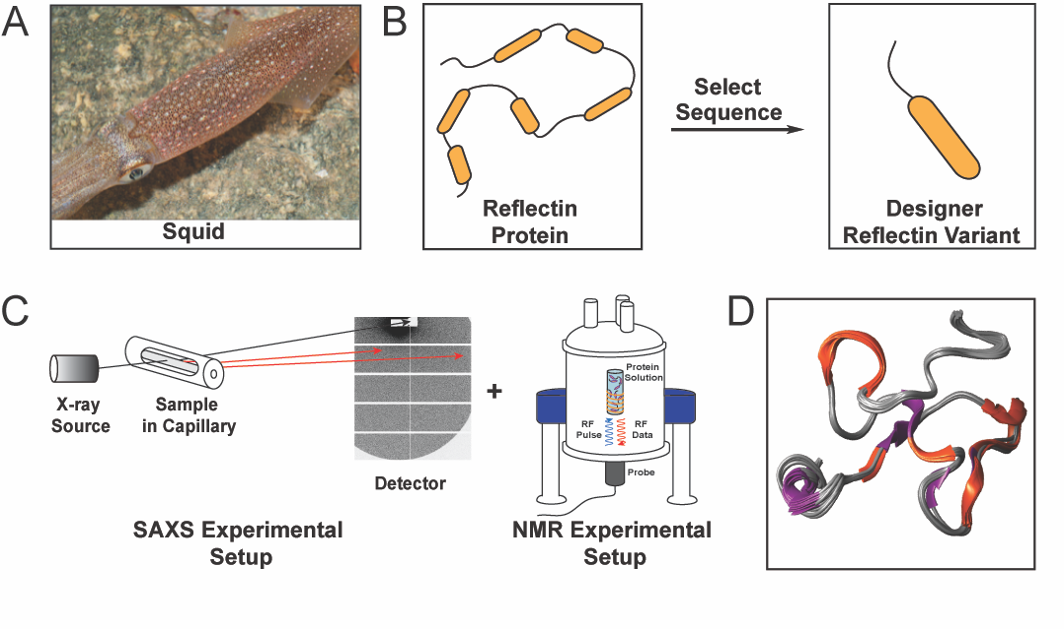
|Reflectins are a family of structural proteins that are known to contribute to the dynamic colouration of cephalopods, such as the squid shown in Figure 1A. These proteins have caught the attention of both scientists and the public because of their role in the colour-changing capabilities of cephalopods and their potential technological applications in electronics, optics, and medicine. As examples, reflectins have been used to fabricate chemically and electrically actuated colour-changing devices and dynamic near-infrared camouflage platforms. However, the understanding of the structures of these proteins was previously hindered by their intrinsic physical properties, such as their extreme sensitivities to subtle changes in the environmental conditions and strong propensities towards aggregation.
In a study coordinated by Prof. Alon Gorodetsky (University of California, Irvine), researchers determined the molecular structure of a reflectin variant, established a precise method to control the variant’s self-assembly and revealed a direct correlation between the protein’s structural characteristics and its optical properties. This scientific work was the result of a large coalition of scientists from across the world, including scientific institutions based in the USA (University of California, Irvine and North Carolina State University), Slovenia (National Institute of Chemistry), Austria (Graz University of Technology), and Italy (Elettra Synchrotron).
The research work started with the analysis of the amino acid sequence of reflectins from several cephalopod species to define a reflectin variant to analyse further (Figure 1B). Having defined the reflectin variant, its shape and folding state was determined by Small Angle X-ray Scattering (SAXS) experiments performed at the Austrian CERIC Partner Facility, while its dynamic 3D structure was determined by 2D and 3D NMR experiments performed at the Slovenian CERIC Partner Facility (Figure 1C). The results obtained from the aforementioned analyses, and complementary data from several biophysical techniques, led to the elucidation of the protein’s molecular structure (Figure 1D).
This work advances the current knowledge of reflectins on multiple distinct fronts, from a better understanding of the mechanisms that enable cephalopods’ camouflage abilities to unlock the potential of reflectins as a structurally tunable material for applications spanning biochemistry, cell biology, bioengineering, and optics.
Below the interview with Atrouli Chatterjee, Mehran Umerani, and Preeta Pratakshya on their research work:



