Deeper insight into platinum-graphene catalysts: towards filtering of exhaust gases and synthesis of energy vectors
|The chemical transformation of carbon monoxide (CO) is one of the most important processes, not only in factories but also in everyday life. Its applications range from the oxidation of poisonous CO to non-poisonous carbon dioxide (CO2) in engine exhaustions, to the so-called “water-gas shift reaction”, where hydrogen, e.g. for fuel cells, is produced by a reaction between carbon monoxide and water. To make these reactions energetically efficient, catalysts based on platinum are widely used. Since platinum is a very expensive and rare precious metal, a strong focus in industrial and academic research is given to the development of catalysts with low platinum content. An approach to optimize platinum catalysts is to exploit the special properties of nano-sized particles on suitable supporting materials like graphene. In this perspective, it is of high importance to understand the interactions between carbon monoxide, the supporting material, and platinum.
The collaboration among three research teams of the University of Trieste, led by Prof. Giovanni Comelli, Prof. Maria Peressi, and Dr. Erik Vesselli, respectively, yielded a breakthrough in understanding a part of these interactions.
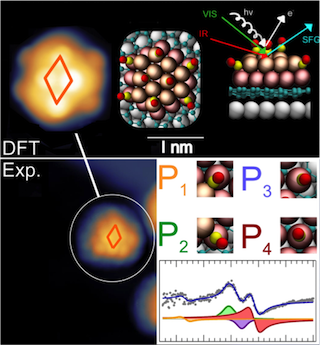
By combining state-of-the art experimental and theoretical approaches with access, within the framework of a CERIC experiment, to instruments available at the Czech Partner Facility, they studied the change of such platinum catalysts on a graphene support when they are exposed to carbon monoxide. They exploited a new near ambient pressure photoelectron spectrometer (NAP-XPS) that allows measurements under almost realistic reaction conditions. Their measurements shed light into details about the mechanisms through which carbon monoxide weakens the connection between platinum and the graphene support. This leads to a coalescence, basically a clotting, of the small particles to larger clusters that might affect the catalytic performance. This effect appears to be size-dependent, and relates with the onset of properties stemming from quantum mechanics due to the small size of the particles. The structure of the particles is also affected, and a peculiar diffusion mechanism takes place, yielding migration of carbon monoxide through the graphene sheet.
These important results mark a step forward in understanding the complex reactions that happens during the catalytic process. This understanding will help to design more efficient catalysts for a wide variety of technical applications.
Original article
-
02.04.2024 | Catalysis, Chemistry
Fabrication of fluorescent MOF micropatterns



