Scientists understood how cytosine methylation shapes G-quadruplex DNA structures
|DNA methylation – where a methyl group is linked to position 5 of cytosine residue – is one of the most important examples of epigenetic gene regulation, with wide-reaching effects from development leading to disease. But what happens when this chemical mark meets the architecture of G-quadruplexes (G4s), which are noncanonical, four-stranded DNA conformations formed by guanine-rich sequences?
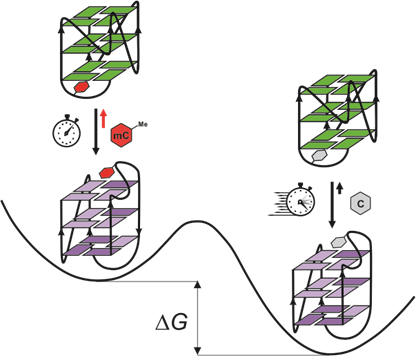
Nataša Medved, Prof Janez Plavec and colleagues of the Slovenian NMR Centre studied a GC-rich region upstream of the P1 promoter of the BCL2 gene, which adopts a major G4 structure with a [3+1] hybrid topology—three guanine strands oriented in one direction and the fourth in the opposite – containing cytosine residues that play critical roles in loop formation and structural stability. By introducing individual 5-methylcytosine (5mC) residues, they found that methylation shifts the equilibrium toward a previously uncharacterized minor G4 structure with a parallel topology. This shift was accompanied by slower folding kinetics and decreased thermodynamic stability of the major structure. Using DAVID and ASKA spectrometers available at the Slovenian CERIC Partner facility (Slovenian NMR Centre at National Institute of Chemistry), researchers characterised this minor G4 structure in atomic detail: it features three G-quartets connected by propeller-type loops and a snapback element that fills a structural gap at the 5′ end, enhancing its stability. Scientists then showed that cytosine methylation effects are context-dependent: the process can redirect folding pathways, modulate polymorphism, and influence how these structures interact with regulatory proteins.
These findings add a new piece to the puzzle of how epigenetic marks, like 5mC, shape the dynamic structure of DNA, with potential consequences for gene expression, cellular identity, and disease. Moreover, understanding how methylation affects G4s formation and stability opens new doors for epigenetic drug design and precision medicine.
ORIGINAL ARTICLE:



