How does ethanol inactivate viruses?
|The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for the ongoing COVID-19 pandemic. Vaccines, masks and disinfectants are among the most effective resources to limit infections. In this regard, ethanol is a widely used and efficient disinfectant employed in healthcare environments and daily hand hygiene. It is effective against enveloped viruses, such as Ebola, influenza, and coronaviruses. However, despite its frequent use, the mechanism of action, and its effect on enveloped viruses, is not fully documented yet.
A study supervised by Prof. Stefan Salentinig (University of Fribourg, Switzerland) responds to this question aiming at elucidating the changes in the structure of the enveloped model virus Phi6 upon interaction with ethanol. Among the experimental techniques employed, Small-Angle X-ray Scattering (SAXS), available at the Austrian CERIC partner facility at the Elettra synchrotron, allowed the investigation of the virus morphology and the nanostructural transformations upon ethanol addition. Experiments showed significant effects on the lipidic envelope rather than the protein nucleocapsid, where the virus’s genetic information is stored. The separation of the envelope from nucleocapsid and the disruption of its structure happened at ethanol concentrations at or above 50% in water, resulting in a complete loss of viral infectivity.
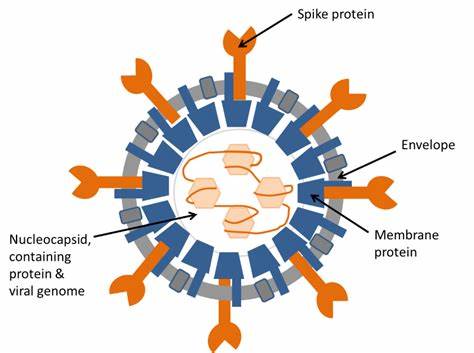
These results confirm the effective action of ethanol as a sanitiser, and its unspecific mode of action is at the base of its wide range of activity. A similar mechanism may also apply to human pathogenic viruses such as Ebola, influenza, and coronaviruses.



